
This atom releases an electron, known as a cation, and acquires a positive charge.Īt the same time, there is a need for an electron-deficient atom, and hence it needs a few more electrons to attain a stable noble gas configuration. While forming an ionic bond, there must be an atom with an excess number of electrons, and it needs to release a few electrons to attain a stable noble gas configuration. Ionic Bond: This is a special type of chemical Bond that is formed by the mutual sharing of electrons by two or more atoms to get a stable physical or chemical state because in a stable state, if the newly formed compound is stable, then it will not further dissociate with any other chemical compound if the newly formed compound is chemically or physically unstable then it will further dissociate until it attains a stable state. The atoms lose and gain the electron and form various chemical bonds to attain a stable noble gas configuration, and it is a normal tendency of an atom to gain a stable noble gas configuration because every atom wants to reach a stable noble gas configuration moreover, they do this to get new and stable chemical and physical properties.

Still, specifically, in this article, we are talking about we are discussing the ionic Bond. What is Chemical Bonding?Ĭhemical Bonding is the formation of chemical bonds among atoms by sharing an electron or by forming different types of bonds like covalent bonds and many other types. But those from a non-scientific background can also relate to it because it is considered the fundamental topic while studying the concept of Chemical bonding.
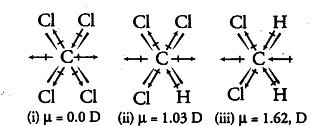
The topic's name indicates that it is related to Chemistry it is for a reader from a science background, it is quite easy for him to understand this concept of bonding because the primary and basic concept while studying chemistry.


 0 kommentar(er)
0 kommentar(er)
